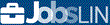Average salary: SG$5,750 /monthly
More stats ...manufacturing operation, new product launches/transfer in a compliant/timely manner, drug substance batch review/release are in full gmp compliance to regulatory standards and ensures quality strategy/continuous improvement are driven in alignment to site objective/s.
About the...
...on Good Documentation practices relating to archival
Be involved in deviation investigations if any
Support data collation and compliance sampling (documentation check) activities for the annual system effectiveness review
Support logistics and activities for audit...
...Maintenance/Equipment, QC) and implementation of Corrective and Preventive Action (CAPA)
Participate in cGMP self-inspections to ensure compliance with internal SOPs and regulatory requirements
Reporting/Documentation
Operate key computer applications related to...
...Manufacturing and Laboratory operations
Support qualification activities
Ensure that the project execution activities are carried in a compliance manner to company policies and regulatory guidelines.
Implement improvements and streamline work processes
What are we...
...Tuas.
The Senior QA Validation ensures that all commissioning and qualification activities under the project scope is designed in compliance with all applicable GSK Quality policies, regulatory requirement, and GMP practices/ procedures in place and in use within GSK Tuas....
...external or internal activities, etc) to execute strategy.
Identify critical spare parts and to deploy adapted actions to ensure compliance and business continuity.
Define the content of maintenance/services contracts with OEM’s and service providers.
Provide/facilitate...
...Site Name: Singapore - Tuas
Posted Date: May 15 2024
Purpose
Responsible for sterility assurance programme and ensure compliance with company polices and current GMP expectations.
Responsibilities
1. QA Oversight
Implement sterility assurance programme on...
...As a QC Associate, you will be responsible for conducting sample testing of incoming materials and/or finished products to ensure compliance with regulatory agencies’ requirements and company standards.
This role will provide YOU the opportunity to lead key activities...
...change management, deviation investigation and production project management to ensure that Value Stream can operate optimally and in compliance with cGMP requirements.
Responsibilities
1. Operations
a) To support Manufacturing Operations in the following areas:...
.../Equipmen , t QC) and implementation of Corrective and Preventive Action (CAPA)
Participate in cGMP self-inspections to ensure compliance with internal SOPs and regulatory requirements
Reporting/Documentation:
Operate key computer applications related to production...
...indicators (KPI) for the supply chain, especially for planning/provisioning monitoring
Ensure that routine audits are carried out in compliance with cGMP & authorized procedures and policies
Ensure timely maintenance of business master data (planning related) in a...
...compendial updates
Keeps up-to-date on regulations/ development of biopharmaceutical QC activities to ensure QC operations are in compliance to applicable corporate, regulatory and external agency regulations
Leads/ manages investigation of non-conformities (events and...
...incubators
Liaise with external vendor for service quotation, escort vendor during service and approve invoice timely
Supports compliance activities:
Performs regular Ll audits in QC
Coordinates and supports all L2, L3 and L4 audits and the associated CAPAs...
...teams
Work in collaboration with Quality Assurance to establish and maintain high standards of cGMP for site SAP activities in compliance with GSK and regulatory standards
Ensure inspection readiness at all times
Support regulatory audits/inspections
Follow-up...
...exceptions) and close the report on time.
Performs data trending and co-ordinates with relevant parties. Reviews QC reports to ensure compliance and promptness of results.
Problem solving
Identifies and reports problems in QC. Solve problems and implement corrective...
...operations.
Key Responsibilities:
Supplier Management
Provide QA oversight of Supplier management activities on site to achieve compliance with company policies and government regulations as assigned.
To participate (Executive 1) and to represent the site (Executive 2...
...trainees upon completion of the programme.
Purpose:
To be primarily responsible for the sterility assurance program and ensure compliance with company polices and current GMP expectations.
Key Responsibilities:
QA Oversight
Implement sterility assurance...
4700 SGD
...gather cost-related data and validate project budgets.
3. Contract Management Support
• Assist in reviewing contracts, ensuring compliance with legal and regulatory requirements.
• Collaborate with legal and procurement teams to resolve contractual disputes and manage variations...