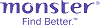Average salary: SG$3,750 /monthly
More statsGet new jobs by email
- ...private sector company with revenues of USD 104.6 billion. Find out more at recgroup.com DESIGNATION : Project Cost and Document Controller Admin RESPONSIBILITIES An independent and diligent individual with precise and meticulous data management and analytical...
- ...medical device company. Collaborate with a diverse and talented team in a supportive work environment. Competitive compensation package and comprehensive benefits. Continuous learning and development opportunities. 5S, Data Management, Quality Control, Teamwork
- ...genuinely improve the world. And that’s the kind of work we want to be part of. We are looking for a Head of Microbial Contamination Control (f/m/d), who will be responsible for developing and implementing proactive Sterility Assurance (SA) and microbial contamination...
- ...of disease together with our Quality Assurance (QA) for Quality Control and Materials Management Internship, 2026: Education... ...existing workflow simplification Perform timely management of documentation Drive continuous improvement mindset What will you learn...
- ...quality issues Handle final approval of engineering drawings and documents, ensuring accuracy and consistency Ensure engineering... ...to-date in compliance with ISO9001 in process and documentation control Identify design shortcomings and recommend improvements...
- ...Define requirements, functional specifications, and testing documentation for various engineering software applications Design and develop... ...interfaces with electrical devices (sending/receiving data, controlling electrical devices via software) Experience working in cross...
- ...abilities with internal and external customers Ability to lead projects under timelines Advanced knowledge in navigating through Controlled Document Management System (DMS) and Quality Management system (Trackwise) Every day, Lonza’s products and services have a positive...
- ...Sampling, aliquoting, distribution and performing of in-process controls Manage the consumables supply necessary for the proper... ...an added advantage High level of conscientiousness and care (documentation, order and cleanliness in the workplace) Technical and Processes...
- ...preparing component specifications, follows work processes to document designs, and prepares materials for design reviews Under minimal... ...tools to document calculations and compare the results in controlled databases Identifies the testing objectives at a component level...
- ...stability testing including testing of intermediates in process control samples and lab operations are accordance with written testing... ...Responsibilities: Sample storage and management. Analytical testing and documentation of API / drug substance / drug product / finished product /...
- ...continuously maintained in a validated state Perform change control assessments for GMP equipment and associated validation activities... ...Perform assigned Quality Systems activities including: Document Management System (DMS), and Track Wise system (Change Control,...
- ...clear technical guidance, both verbal and written, in support of Documented Corrective Trends (DCTs) and Non-Conformance Investigations (... ...equipment. Ensure incoming inspection of medium- and low-risk Controlled Supply Items (CSIs), including review of material certificates,...
- ...and process validation relevant to compounding. Ensure proper documentation practices (cGDP) are followed for all production and cleaning... ...processes and equipment. Drive initiatives to reduce waste, control costs, and improve overall equipment effectiveness (OEE). Manage...
- ...complaints investigations. To ensure good 5S standards, batch documentation completeness in accordance to procedures for DSM Flex lines,... ..., Advanced / Higher / Graduate Diploma Experience in GMP controlled manufacturing/production environment is an advantage Able to...
- ...readiness at all times by maintaining audit-compliant systems, documentation, and practices. Lead the self-inspection program, including... ...and monitor key quality metrics (e.g., deviations, CAPAs, change controls), identifying trends and proposing process enhancements....
- ...administration/tracking/reporting, compliance and continuous improvement of the MoC system. Responsible for administration, control, documentation, maintenance and management of recipe systems in accordance with MoC procedures. Support new product, raw materials,...
- ...associated with Compounding Perform and review all necessary documentation as required and in accordance with Good Documentation Practice... ...and verbal communications Ensure medium and low risk level controlled supply item (CSI) incoming inspection, review of Material...
- ...will develop contractual strategy and framework to manage vendors / contractors and corresponding contracts. Project Engineering & Control: You will manage the investment budget and project portfolio for the site, lead investment review committees, and develop and execute...
- ...technical projects. Utilize technical influence and strong scientific knowledge to drive Technology Transfer and monitor state of control of process, provide direction and oversight on resolution of complex issues, introduction of new bioprocessing technologies and site...
- ...Job Duties Working under close supervision, sets up, adjusts, and operates a variety of machine tools which may include numerical control/computerized numeric control lathes, mills, drills, computerized numerical control/numerical control and/or manual machines. May be required...
- ...Instrument list, IO list, Instrument datasheet, Functional description/Control narrative, Sequence diagram, Control loop diagram, Alarm... ...Junction box, Cable tray routing drawing, Control system related documents and Electrical-Instrument interfacing documents and other Construction...
- ...to support the business requirements. Analysis of bulk product according to Standard Operating Procedures and Test Methods. Documentation of results in accordance with current Good Manufacturing Practices (cGMP). Problem solving of analytical methods as well as troubleshooting...
- ...design datum (Object amount) Attend and report in project and section meeting Complete the assigned tasks for project drawing or document. Prepare & Review marine machinery/piping calculation, specification and vendor's drawing Prepare & Review marine machinery/...
- ...providing technical input and support where required. Use a strong understanding of manufacturing processes, equipment capabilities, control logic, and trend analysis to identify and resolve recurring deviations and improve process robustness. Develop and implement...
- ...building of a digital learning culture in REC when required. Ensure learning activities and records are properly administered and documented in conformance with the L&D policy, Grants and Audit requirement. Support all related ISO & Quality audits to ensure compliance....
- ...minimal supervision. Able to perform overtime when required. What competencies will you need? Ability to read and interpret documents such as safety rules, operating and maintenance instructions, and procedure manuals. Ability to write routine reports and correspondence...
- ...of drawings to identify errors, inconsistencies, or discrepancies and make necessary corrections to maintain quality standards. Documentation and Record-Keeping: Manage, maintain, and archive engineering drawings and documents through our “check, approval and release”...
- ...site's GxP training management process. Appointed site's admin for Veeva Quality Learning Management System, manage the user access control and responsible for all training related requests such as processing Training Change Request (TCR), Training Requirement Impact...
- ...Singapore, primarily based in the Science Park facility. Job Responsibilities: Adhere to EHS rules and regulations at all times. Document results in accordance with Lonza policies and current Good Manufacturing Practices (cGMP). With support from Manager/Study Lead,...
- ...lenses are manufacturing to the best quality level possible. Perform activities in compliance to Good Manufacturing Practices in a controlled and regulated environment. To ensure compliance to Data Integrity such that information are attributable, legible,...